Le resumo una carta que envió el Dr. Hooman Noorchashm, PhD a la FDA, Pfizer y Moderna, que quizás conteste a su pregunta.
Tiene un enlace de la trayectoria profesional del Dr.Hooman Noorchashm por si quiere validar su conocimiento.
Hooman Noorchashm, MD, PhD | Expert Medical Contributor
Enlace con la carta completa.
A Letter of Warning To FDA And Pfizer: On The Immunological Danger Of el bichito-19 Vaccination In The…
"El receptor ACE-2 del endotelio es el portal de entrada del bichito en las células endoteliales, y parece que la lesión endotelial por el bichito o por la reacción inflamatoria que incita, es la razón por la que muchos pacientes de el bichito-19 experimentan complicaciones tromboembólicas.
Así que es una cuestión de certeza que los antígenos virales están presentes en el revestimiento endotelial de los vasos sanguíneos en todas las personas con infección activa o reciente, independientemente de si son sintomáticos o convalecientes.
Escribo para advertir que es un pronóstico inmunológico casi seguro que si los antígenos virales están presentes en los tejidos de los sujetos que se vacunan, la respuesta inmune específica al antígeno desencadenada por la banderilla se dirigirá a esos tejidos y causará inflamación y daño tisular.
Y lo que es más pertinente, cuando los antígenos virales están presentes en el endotelio vascular, y especialmente en ancianos vasculópatas y frágiles, la respuesta inmunitaria específica al antígeno incitada por la banderilla es casi segura que causará daños en el endotelio vascular. Dicha endotelitis dirigida por la banderilla provocará con toda seguridad la formación de coágulos sanguíneos con el potencial de provocar complicaciones tromboembólicas importantes, al menos en un subconjunto de dichos pacientes. Si la mayoría de los pacientes más jóvenes y robustos podrían tolerar tal lesión vascular, muchos vasculopáticos ancianos y frágiles no lo harán".
Coste vs Beneficio, siempre es una difícil elección.
Muy buena información, gracias. El mismo médico da pautas sobre la conveniencia de ser medicado:
The Safest Way to Get Your el bichito-19 Vaccine: #ScreenB4Vaccine
ellow Citizen,
In a recent letter to the FDA and
Pfizer leaderships, I warned of the serious immunological danger, which the el bichito-19 vaccine might pose to those infected or recently convalescent from el bichito-19 infection.
You may read my public letter of warning
HERE.
My very specific concern stems from the fact that the SARS-CoV-2 bichito is known to accumulate in the inner lining of blood vessels — the so-called
endothelium. So if a person with a recent or active el bichito-19 infection is vaccinated, the highly effective and antigen specific immune response incited by the vaccine will, very likely, attack the inner lining of the blood vessel and cause damage, leading to blood clot formation. This could result in major serious problems like strokes and heart attacks, at least in some people. I project that this risk will be highest in the elderly, the infirm and those with cardiovascular disease.
Of course, using this nearly certain scientific prognostication, I will state that any anatomic location in the body where the viral antigens may be present, is also likely to be targeted and damaged by the vaccine immune response.
Here I am writing this simple guidance, which anyone can use to keep themselves and their loved ones as safe as possible from a potential vaccine side-effect.
As a disclaimer, here I am not addressing the issue of allergic reactions to the el bichito-19 vaccine. Because the data from Pfizer and Moderna on vaccine allergies are robust. These vaccines were shown by the Pfizer and Moderna trials to be no more prone to causing allergies that our other commonly used vaccines. So the probability of a vaccine allergic reaction to the el bichito-19 vaccine is very low — and the appropriate mitigation steps are already in place to treat any persons who experience classical vaccine allergies, when they receive the el bichito-19 vaccine. You should not be weary of these — unless we have a history of serious or severe past allergic reactions, in which case the likely recommendation is to forego vaccination.
The real safety concern I am addressing here is that vaccinating individuals who might already have viral antigens in their bodies, at the time they get vaccinated, might trigger a dangerous inflammatory reaction in the tissues where the antigen is localized. And in the case of the elderly, the infirm or those with significant cardiovascular disease, such vaccine incited inflammatory reactions could prove deadly.
But who are these individuals who are almost certain to be carrying SARS-CoV-2 antigens in their tissues?
They are the recently convalescent el bichito-19 patients and those with active, symptomatic or asymptomatic, infections.
So, in order to ensure that you are maximally safe when receiving your el bichito-19 vaccine, you should determine if you fall into one of these categories.
How?
By Screening yourself or your loved ones for evidence of el bichito-19 infection soon before you get vaccinated.
There are three ways to do so:
- el bichito-19 PCR assay for Viral mRNA/DNA: This is a very sensitive method for detecting the presence of the bichito in your body. depending on the center and your geographic location in the US, this test result can take anywhere from 24–72 hrs to return.
- el bichito-19 Rapid Test for Viral Protein: This is also a sensitive test for detection of viral antigen. It is less sensitive than the PCR assay, but can be done rapidly within 10–15 minutes — like a pregnancy test — to achieve a reasonable certainty about your carrier status.
- el bichito-19 Antibody test: This is a blood test that detects IgM and IgG antibodies specific for SARS-CoV-2. A positive test indicates that the person was highly likely to have been exposed to the bichito >7–10days prior to the blood test.
It is safest to screen ALL vaccine candidates with the Antibody test (#3) to establish if there is any possibility of past exposure to the bichito. In addition, because the antibody test is unlikely to be positive in the case of recent infections (i.e., <7–10 days), using either the PCR Screen or Rapid Test immediately prior to vaccination is safest.
It is critical that the elderly, the infirm and any persons with cardiovascular disease be screened with at least one, preferably two (as described above), of these assays immediately prior to vaccination— in order to maximally mitigate against the possibility of activating a dangerous inflammatory response.
Certainly, if you are el bichito-19
“Long Hauler”, with symptoms persisting for over 2 months, you ought to consider delaying your vaccination until resolution of symptoms — this, because the “Long Hauler” syndrome is highly likely to be the result of an ongoing inflammatory reaction in your body. Vaccination, any kind of vaccination, could exacerbate this syndrome — so best to delay until resolution of symptoms.
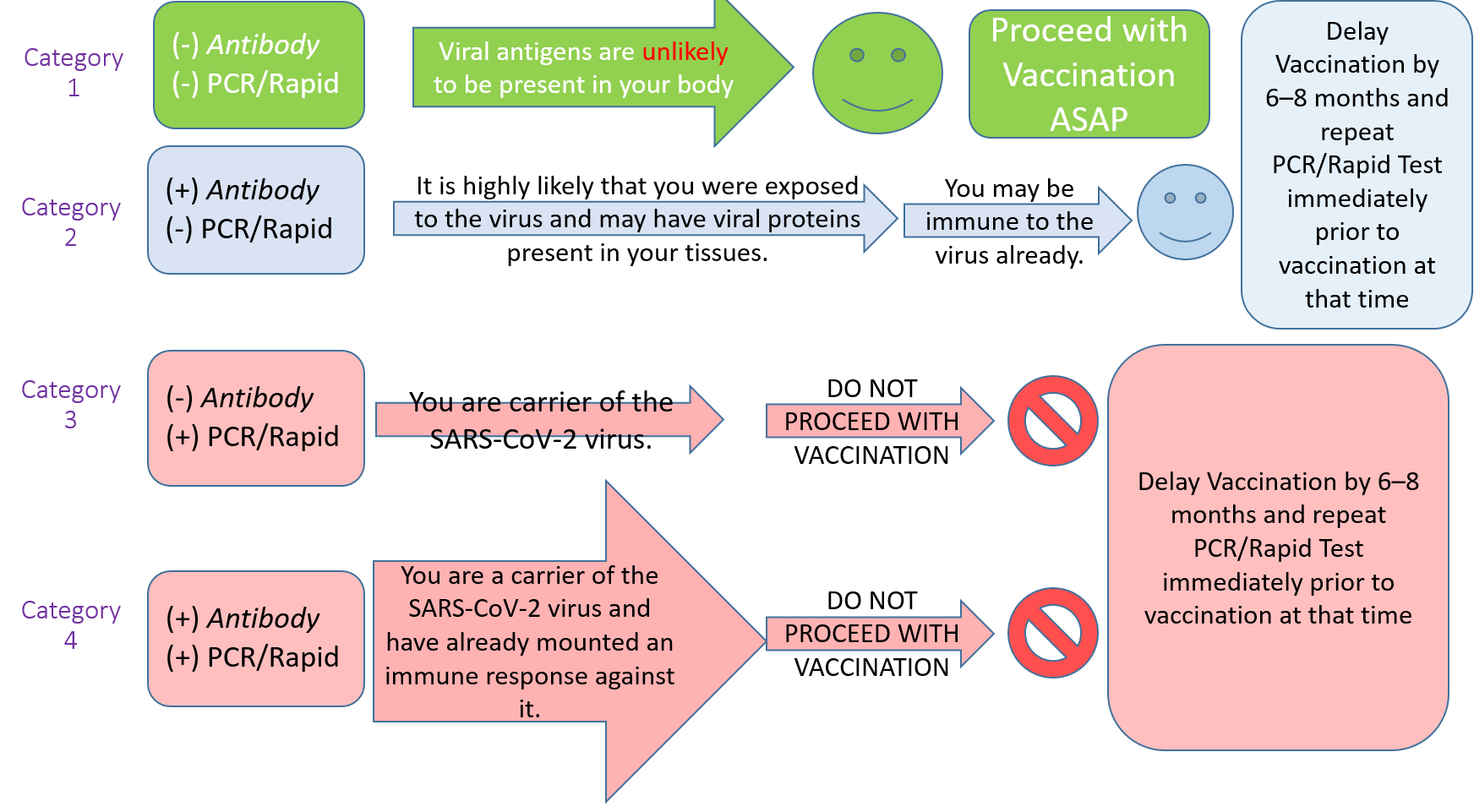
Here is a step-by-step roadmap on how to safely proceed based on the obtained results:
- Antibody Test Negative, PCR/Rapid Test Negative: Viral antigens are unlikely to be present in your body. Proceed with Vaccination ASAP.
- Antibody Test Positive, PCR/Rapid Test Negative: It is highly likely that you were exposed to the bichito and may have viral proteins present in your tissues. You may be immune to the bichito already. Delay Vaccination by 6–8 months and repeat PCR/Rapid Test immediately prior to vaccination at that time.
- Antibody Test Negative, PCR/Rapid Test Positive: You are carrier of the SARS-CoV-2 bichito. DO NOT PROCEED WITH VACCINATION. Delay vaccination by 6–8 months and repeat PCR/Rapid Test immediately prior to vaccination at that time.
- Antibody Test Positive, PCR/Rapid Test Positive: You are a carrier of the SARS-CoV-2 bichito and have already mounted an immune response against it. DO NOT PROCEED WITH VACCINATION. Delay vaccination by 6–8 months and repeat PCR/Rapid Test immediately prior to vaccination at that time.
If you fall in Category 3 or 4, you may have symptomatic el bichito-19 disease or be asymptomatic. Proceed as amows:
A) If you are asymptomatic: Repeat your PCR/Rapid test in 6–8 months. If negative, proceed with vaccination.
B) If you have symptomatic el bichito-19 disease: Repeat your Antibody test amowing convalescence and if positive, you are highly likely to be immune. This is the expected finding in the vast majority of people who’ve had previous el bichito-19 disease. In this case, you may either choose to forgo vaccination, or you may receive the vaccine. If you choose to be vaccinated, repeat the PCR/Rapid Test immediately prior to vaccination and proceed if negative.
The overall concept is that if you are a carrier of the bichito, either by PCR or the Rapid test, you are safest delaying your vaccination by 6–8 months — in such a case, if you developed symptomatic disease, and are Antibody positive, you may also consider forgoing vaccination, because you have natural immunity. With asymptomatic infection, even if the Antibody test turns positive, it is safest to consider getting the vaccine. The basic premise being that symptomatic natural infection, while not the preferable way to achieve immunity, is itself a very powerful way to develop immunity.
A proposed safety algorithm to protect the already infected from a potential el bichito-19 vaccine reaction (Graphic by N. Omrani).
Overall, I am confident that the el bichito-19 vaccines developed by Pfizer and Moderna are extremely effective at stimulating a protective immune response to SARS-CoV-2. BUT, it is absolutely essential that every citizen be maximally safe also.
It is highly likely unsafe to vaccinate any persons with viral antigens present in their tissues — especially, the elderly and the infirm with cardiovascular disease — because so doing risks inciting a dangerous inflammatory response in these tissues.
Dear citizen, please do not doubt that the el bichito-19 vaccine can protect you from illness and save our nation from the existential peril we confront — BUT know that you can and must act to keep yourself and your loved ones safe from a potential dangerous vaccine side-effect.
Remember this analogy from driving an automobile: Driving has a risk of harm in car accidents. But driving an automobile is absolutely essential to our modern way of life — AND the vast majority of cars do not get into accidents on any given day. So, we do not cast doubt on the need for driving cars because of the definite risks involved. Instead, we act rationally (and scientifically) to mitigate these risks by amowing good traffic regulations, defensive driving, wearing seat belts, installing air bags, using ultrasound and camera detection systems. We drive, but we act rationally using best information to reduce risk.
The el bichito-19 vaccine is absolutely essential to rescue us from this pandemic — so we all need to be vaccinated. But we MUST do so as safely as possible by mitigating all known and projected risks.
Remember, the el bichito-19 vaccine is absolutely critical for us to be able to survive this pandemic and minimize deaths moving forward. But, we must do everything scientifically possible to mitigate against the risk of harm from the vaccine itself….My recommendation as a physician and immunologist is to #ScreenB4Vaccine for maximum safety — in order to exclude the possibility of directing the vaccine initiated immune response to tissues harboring viral antigens.
I write you in friendship and defense of public health in the United States and globally.
Hooman Noorchashm MD, PhD
noorchashm@gmail.com